A naphtha tank bottom cut water tank bypass metal hose leaked in a refinery. The hose is used for extracting crude oil from the bottom of the tank. The medium in the tube is normal temperature naphtha produced by another petrochemical plant, and the bottom pressure is hydrostatic pressure. The height of the liquid in the tank is about 17 m and the pressure is about 0.12 MPa.
Physical and chemical inspection
1 Macroscopic examination
As can be seen from Figure 2, the metal hose is not mounted horizontally. Remove the leaking hose and remove the surface metal mesh. Observe that the bellows is visible in about three-quarters of the area, and still retains metallic luster. About one-fifth is yellow-brown.
The corroded area is directly under the bellows, and there are holes that have been eroded on the outside. The inner surface of the bellows also has a large number of uncorroded pits. It can be determined that the corrosion is gradually extended from the inner surface of the bellows to the outer surface.

Figure 2 Metal hose installation location
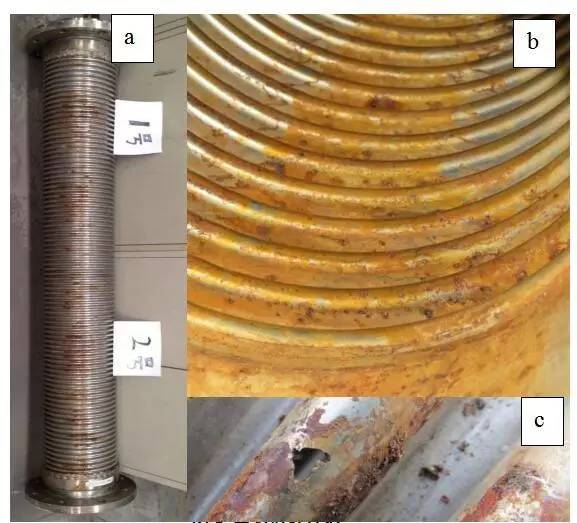
Picture 3 Macro corrosion
(a) Outer surface topography
(b) Internal surface topography
(c) External surface corrosion perforation
2. Microscopic observation
2.1 Metallographic examination
The bellows is sampled at the perforation of the bellows, and the hole is opened along the center of the perforation. After the obtained sample is inlaid, the metallographic examination of the bellows is performed according to the metal microstructure inspection method of GB/T 13299-1991.
It can be seen from Fig. 4 that the inner wall thickness of the bellows is gradually thinned from the parallel section to the trough direction, and corrosion holes are formed at the troughs, and the perforations are expanded from the inside to the outside, and a plurality of corrosion pits are visible on the inner surface.
It can be confirmed from Figs. 5 to 8 that the material for processing the bellows is not subjected to solution treatment.
Corrosion mostly propagates along the deformed martensite part, especially the dislocation extends into the crystal. The metallographic examination near the edge of the corrosion pit shows that most of the deformed martensite is shown in Fig. 6.

Picture 4 Corrugated pipe perforated cross section metallographic full picture
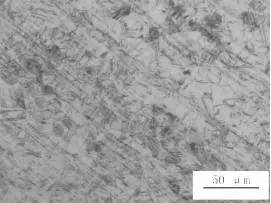
Picture 5 Metallographic structure of the corrugated pipe cross section near the valley

Figure 6 Metallographic structure of the inner surface of the edge of the etch pit

Figure 7 Metallographic organization of small denatured areas
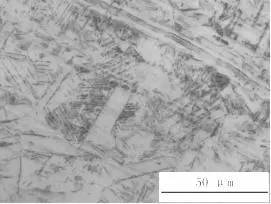
Picture 8 Metallographic organization of the trough region
2.2 Scanning electron microscopy
Sampling at the perforation of the bellows, opening the hole along the center of the perforation, cleaning the corrosion product covered by the surface of the etch pit with dilute sulfuric acid, and making a sample for observation by scanning electron microscopy (SEM). The crystal at the edge of the etch pit can be seen from Fig. 9. The particles undergo different degrees of corrosion. The dislocations and slip lines formed during the deformation of the tube are clearly visible.

Figure 9 Corrosion pit edge topography
Energy spectrum (EDS) was used to analyze the composition of corrosion products in typical areas around the pit. The results are shown in Table 1.
Table 1 Energy spectrum analysis results of typical parts of bellows corrosion penetration
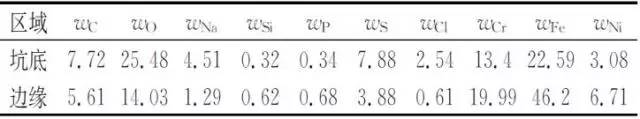
It can be seen from Table 1 that the corrosion product contains chlorine and sulfur in addition to the constituent elements of the metal material itself, the common elements in the naphtha and the oxygen in the air. Among them, the bottom of the pit measured the highest content of chlorine and sulfur.
A corrugated tube was used to etch the perforated edge portion to prepare a metallographic examination sample. Using the scanning electron microscope, the cracked microcracks were found on the etched pit side of the ground metallographic sample, and the microcracks extended toward the inside of the grain along the dislocation flow line after deformation. The energy spectrum analysis was carried out in the area of the crack tip selection box, and the wCl was found to be 1.46% and the wS was 3.58%.
The energy spectrum analysis of the products attached to the inner surface of multiple corrosion pits of metal hoses revealed that chlorine was present in many places and was accompanied by the presence of sulfur. In particular, the wCl was measured at the crack tip to be 1.46%.
The inner wall of the bellows was peeled off and collected for X-ray diffraction (XRD) analysis, and the corrosion product was analyzed to contain NaCl, as shown in FIG.
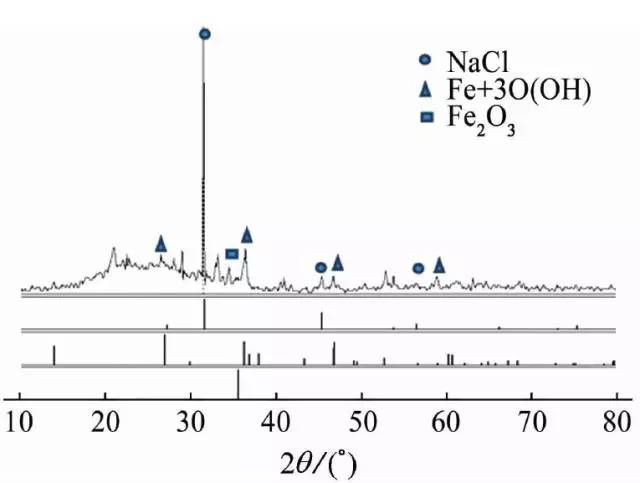
Figure 10 XRD analysis of corrosion products
3. Chemical composition analysis
The inner surface of the corroded perforated pit is attached with a large amount of corrosion products, and several serious corrosion pits are selected on the obtained metal hose, and the topography of the corrosion pit and the side product are photographed, and the corrosion product components are recorded by EDS. The EDS analysis of the attached product on the inner surface of the bellows.
The corrosion pit bottom product was analyzed in the form of particles. The content of chlorine element in the bottom of the corrosion pit was 0.61%, the content of sulfur element was 3.88%, the scanning area and scanning line were shown in Fig. 11, and the analysis data are shown in Table 2. Sampling at the perforated portion of the bellows, removing the table after crushing
Surface oxide layer, made of samples for spectral analysis, according to GB/T11170-2008 "Stainless steel multi-element determination of spark discharge atomic emission spectrometry (conventional method)" for the spectral analysis of the obtained bellows, the results are shown in the table 2.
Table 2 Corrugated tube chemical composition
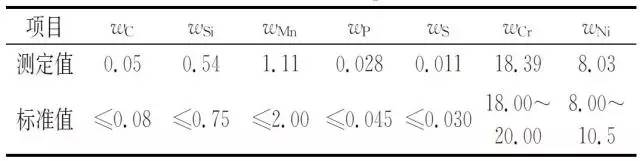
As can be seen from Table 2, the bellows composition is in accordance with ASMESA 240 "Pressure Vessels and Heat-Resistant Chromium and Chrome-Nickel Stainless Steel Plates, Sheets and Strips for General Use".
4. Analysis of naphtha precipitation water
The bottom water sample of the naphtha E tank was taken at 13:00 on November 7, 2014, and the sulfide and chloride ion contents were analyzed. The results are shown in Table 3. It can be seen from the data in Table 3 that the concentration of chloride ion in the bottom of the naphtha tank bottom is 876 mg/L, which is basically consistent with the monitoring in the early stage of the workshop, while the sulfide mass concentration is stable at 330 mg/L.
Table 3 Analysis of OSBL TANK naphtha E tank water sample 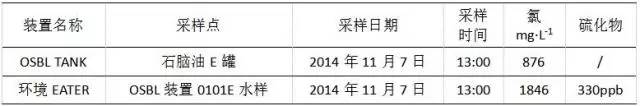
Comprehensive analysis
The function of the cracked metal hose according to the process design is to turn the oil at the bottom of the oil storage tank to the top of the tank to make the oil quality in the tank uniform. However, from November 2013 to November 2014, the storage tank did not perform this work and was in a stable state filled with medium. The medium in the tank is naphtha. When the naphtha is stored in the oil storage tank for a long time, water will be deposited, and the layered water needs to be discharged through the water cut tank. During the drainage process, the water at the bottom of the tank will enter the metal hose, and a small amount of water will be deposited in the naphtha remaining in the metal hose for a long time. The water accumulates in the hose for a long time to form a corrosive environment. A large amount of chloride ions are dissolved in the water, the material is exposed to a corrosive environment, and the area covered by the surface passivation film is broken, that is, the passivation film is destroyed. The outer surface of the failed metal hose is covered with a layer of wire, which is a bellows obtained by cold stamping deformation. During the cold stamping deformation process, the material has great structural stress, and a large amount of deformation is found in the metallographic examination. Induced martensite, and the deformation induced martensite main deformation in the bellows is the top of the peaks and troughs. These regions have large dislocations, and the dislocation lines are concentrated outcrops, which accelerates the failure of the passivation film and the occurrence of corrosion. , eventually leading to corrosion perforation.
Sulfur is detected in the corrosion product, and the hydrolyzed product of the sulfide acts as an activation, further hindering the repair of the oxide film by the chloride ion breakdown region. The hydrolysis of the sulfide also causes corrosion of the bellows and yellow corrosion products. In a primary battery consisting of a sulfide, a chloride ion solution, and a stainless steel matrix, the sulfide in the material dissolves with the sulfide and can be reacted as follows to form H+:
MnS+4H2O Mn2++SO4-+8H++8e
H2S is also generated in different corrosive environments, which also hinders the re-passivation of small pits, allowing them to continue to dissolve and become a source of pitting.
Conclusions and recommendations
1.
The metal hose is cold-worked, and a large amount of deformation inside the material induces martensite, and the deformation also causes the dislocation to appear out at the maximum deformation. In the aqueous solution containing chloride ions, the passivation film of the material itself is broken down, and the presence of sulfides promotes the occurrence of corrosion, eventually leading to corrosion perforation;
2.
The solution forming bellows is solution treated to reduce the amount of deformed martensite and reduce the pitting corrosion sensitivity of the material;
3.
Through the anodizing treatment, a chromium-rich protective film is formed on the surface, which can greatly improve the pitting potential, or can also be surface aluminized.
















 RCCN WeChat QrCode
RCCN WeChat QrCode Mobile WebSite
Mobile WebSite